MedPhab is Europe’s first Pilot Line dedicated to manufacturing, testing, validation and up-scaling of new photonics technologies for medical diagnostics enabling accelerated product launch with reduced R&D costs.
- About
- Overview
- Login Area
About
MedPhab serves as Europe’s first Pilot Line dedicated to manufacturing, testing, validation and up-scaling of new photonics technologies for medical diagnostics and within its broad remits enables accelerated product launch with reduced R&D costs.
MedPhab brings the high-quality infrastructure and extensive know-how, both within an easy reach of the SME’s and other European Industry. A globally unique feature of MedPhab is its operation in the medical domain setting requirements to meet regulations. The competitive differentiation is the seamless operation between ISO13485 certified companies and RPOs when single-entry point can serve SMEs operating at different TRLs. Professional customer interface will be operated by staff with extensive experience in the field of medical devices. The technology readiness and MedPhab operation process will be validated by:
- A use case validation program, upscaling and demonstrating performance of 5 innovative medical diagnostics products
- A Demo Case Open Calls program enabling early adoption of the technologies by external user, demonstrating the pilot line services and validating the open access business model.
Three IPIC groups participate in MEDPHAB (Biophotonics, Packaging and Business Development). The project has received 15M€ funding from Horizon 2020 the European Union Framework Programme for Research and Innovation under grant agreement no. 871345 and is coordinated by VTT (Fi).
The other partners are Joanneum Research (At), IMEC (Be), CSEM (CH), Philips (NL), Jabil Circuit (At), Screentec (Fi), III-V Lab (F), Stryker (IE), POLAR (Fi), Radisens (IE), MyCartis (BE), Genspeed Biotech (At), NKI (NL), EPIC (F), AMIRES (CZ). The project runs from Jan 2020-Dec 2023.
Overview
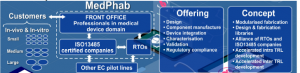
In addition to SMEs and RPOs, large and innovative European companies spearhead the actions. The concept, which differentiates MedPhab from other pilot lines and available services, relies on medical driven application domain and accelerated production model provided by the modularised fabrication and established production libraries.
Depending on customer needs and required TRL, the work is distributed between ISO13485 (standard for medical device manufacturing) certified companies and RTOs. The possibility to realise devices at different TRL using same/compatible production equipment can greatly accelerate time to market by eliminating the need to redevelop processes, reprogram equipment, or retrain personnel.
Additionally, the participation of companies with ISO13485 standardized manufacturing ensures the seamless transition from pilot line production to up-scaled manufacturing without a need for changing service providers.